Crude Column & Distillation Column.
The Crude Column splits raw crude into several product streams that are further processed in other units in the refinery: Light naphtha to light ends recovery. Heavy naphtha to reforming. Kerosene to hydrotreating. Distillate (sometimes called diesel) to hydrocracking or hydrotreating..
INDEX
1. What is Column in Oil and Gas?
A distillation column is a tall vessel in which crude oil is heated and separated into its components. The first process in the refining of crude oil is fractional distillation which is carried out in a tall steel tower known as a distillation column.
2. What is Crude Column?
Crude units are the first units that process petroleum in any refinery. There objective is to separate the mixture into several fractions like naphtha, kerosene, diesel and gas oil. A schematic diagram of an atmospheric crude fractionation unit is shown in Figure
3. What is Distillation in Refinary?
A distillation column is a tall vessel in which crude oil is heated and separated into its components. The first process in the refining of crude oil is fractional distillation which is carried out in a tall steel tower known as a distillation column.
Oil refineries use two main types of distillation columns. Oil refineries use two main types of distillation columns.
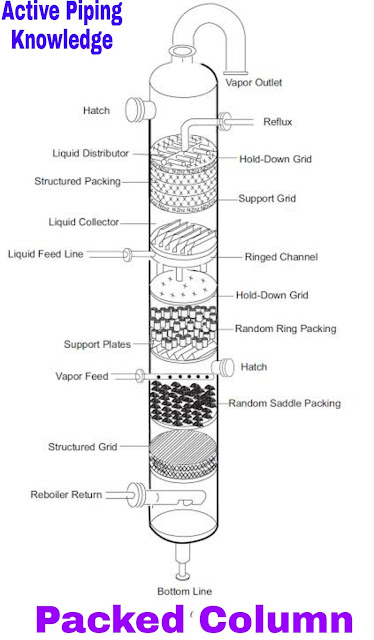
1. Packed Type Columns
2. Tray Type Column
4. What is Crude Topping column?
Essentially they are atmospheric distillation units that produce hydrocarbon boiling point cuts from resid to LPG. A topping unit is an inexpensive, technically simple means to enhance the value of crude oil near the source of production.
5.What is Crude Unit?
Crude units are the first units that process petroleum in any refinery. There objective is to separate the mixture into several fractions like naphtha, kerosene, diesel and gas oil.
6. What is crude distillation unit?
The crude oil distillation unit (CDU) is the first processing unit in virtually all petroleum refineries and one of the most critical. The CDU distills the incoming crude oil into various fractions of different boiling ranges, each of which are then processed further in the other refining processing units.
7. Basic Knowledge of Distillation Column and it's Type ?
- Distillation column is a critical piece of equipment in the refining of crude oil. It acts very much like a still, separating product into its different chemical components based on differences in volatility. Oil refineries use two main types of distillation columns. They are:
Packed Type Columns –
A packed distillation column is a vertical tower packed in sections with ceramic raschig rings, ceramic saddles or steel pall rings. The packing material is used to increase the surface area for mass transfer between gas and liquid phases during the distillation process.
The process stream is heated before entering the column, which causes partial vaporization. As it moves up the tower, the vapor cools. Light components will continue to rise and heavier components will condense and fall to the bottom as liquid.
A reboiler at the bottom of the column adds heat to create vapor, which strips light components out of the liquid. Packed towers are typically onlyused to produce a top and bottom product.
Tray Type Column –
Tray type distillation columns operate on the same principle as packed columns; however, instead of using packed material they use trays situated at various heights within the tower. The trays have a weir to maintain a layer of liquid across the surface.Hot vapor bubbles through the liquid to strip out light components. As the liquid flows over the weir, it is directed to the tray below.
There are three main types of trays in use:
1. sieve trays
2. valve trays &
3. bubble cap trays.
Bubble cap trays generally achieve the most efficient separation of product components. The bubble cap design distributes the vapor more extensively, forcing it to bubble through the liquid flowing across the tray.
In general, tray type columns are more efficient than packed type columns, but are also more expensive. They do, however, facilitate multi-stage separation of products via side streams in the tower, and are therefore much preferred for crude oil distillation.
8. How Column Work?
Distillation is a process used to separate a mixture of two (or more) components into its virgin state by heating the mixture to a temperature between their respective boiling points.
For example, at atmospheric pressure, water boils at 212ºF and ethanol boils at about 176ºF. If the mixture of water and ethanol is heated to about 195ºF, the ethanol will boil and change into vapor which is then collected and condensed. The water will separate and remain as a liquid.
A fractionating distillation column is used to make this process more efficient. See the picture below to better understand how columns work.
The distillation column is made up of a series of stacked plates. A liquid feed containing the mixture of two or more liquids enters the column at one or more points.
The liquid flows over the plates, and vapor bubbles up through the liquid via holes in the plates.
As liquid travels down the column, the vapor comes in contact with it several times due to the multiple plates – a critical process in distillation columns.
The liquid and vapor phases are brought into contact because as one molecule of higher boiling material converts from vapor to liquid phase by energy release, another molecule of the low boiling material utilizes the free energy to convert from liquid to vapor phase.
The base of the distillation column contains a large volume of liquid consisting mostly of the liquid with higher boiling point (in our example, this would be water). Out of the base flows some of this liquid, some of which is heated in the re boiler and returned to the column. This is called the boil up.
Some vapor escapes from the top of the column and is returned to a liquid state in the condenser. Some of this liquid is returned to the column as reflux, and the remainder is the top product or distillate. Vapor and liquid phases on a given plate approach thermal pressure and composition equilibrium to an extent depending upon the efficiency of the plate.
In essence, the hot mixture is pumped into the bottom. The tower acts as a heat exchanger, removing heat from the vapors as they rise. Some of them condense back into liquids and fall back down the column.
The temperature gradually decreases as you go up the column. Different groups of hydrocarbons condense at different heights – the heaviest at the bottom, the lightest at the top. The final product is in its virgin state.
9.Why are Distillation Column is Important?
A distillation column is an essential item used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on the differences in volatilities. Fractionating columns are used in small scale laboratory distillations as well as large scale industrial distillations.
10.What is the purpose of Column in Oil and Gas?
A distillation column is a tall vessel in which crude oil is heated and separated into its components. The first process in the refining of crude oil is fractional distillation which is carried out in a tall steel tower known as a distillation column.
11.What is the process of crude Distillation Column?
A crude oil distillation process is the process of heating crude oil and passing the vapor through a vessel to separate out different compounds, known as fractions. In the crude oil distillation process, the crude oil is heated to 400°C and separated into fractions on 30-50 fraction trays in a distillation column.
12.How many steps in Fractional distillation column?
A fractional distillation of crude oil carries out several steps:
1. Heating the mixture of the substances of crude oil (liquids) with different boiling points to a high temperature. Heating is usually done with high pressure steam to temperatures of about 1112 degrees Fahrenheit / 600 degrees Celsius.
2. The mixture boils, forming vapor (gases); most substances go into the vapor phase.
3. The vapor enters the bottom of a long column (fractional distillation column) that is filled with trays or plates. The trays have many holes or bubble caps (like a loosened cap on a soda bottle) in them to allow the vapor to pass through. They increase the contact time between the vapor and the liquids in the column
and help to collect liquids that form at various heights in the column. There is a temperature difference across the column (hot at the bottom, cool at the top).
4. The vapor rises in the column.
5. As the vapor rises through the trays in the column, it cools.
6. When a substance in the vapor reaches a height where the temperature of the column is equal to that substance's boiling point, it will condense to form a liquid. (The substance with the lowest boiling point will condense at the highest point in the column; substances with higher boiling points will condense lower in the column).
7. The trays collect the various liquid fractions.
8. The collected liquid fractions may pass to condensers, which cool them further, and then go to storage tanks, or they may go to other areas for further chemical processing.
Welcome to Our Blog Active Piping Knowledge.
I am Afzal an experienced piping engineer from the last 8 years. Through this platform, I will share my experiences and knowledge with you in an innovative way. So be with me for the next couple of years!
For any Query afzalpipingknowledge@gmail.com.












Comments
Post a Comment